



Achieve Accurate Study Data Collection and Enhance Study Data Quality with eCOA and ePRO
ePRO (electronic patient-reported outcome) and eCOA (electronic clinical outcome assessment) technology have become increasingly important tools in clinical trials. Here are some of the ways in which these technologies are used:
-
- Collecting patient-reported outcomes such as symptom severity, quality of life, and treatment satisfaction. These data can help to evaluate the efficacy of new treatments and inform clinical decision-making.
- Enhancing data quality and accuracy by reducing errors in data entry, missing data, and illegible responses, resulting in higher quality and more accurate data.
- Improving patient engagement and compliance by making it easier for patients to participate in clinical trials by providing user-friendly interfaces and making it more convenient to submit data. Additionally, real-time feedback and alerts can help to improve patient compliance and engagement.

Introducing ActiGraph eCOA & ePRO Solutions
ActiGraph is a leading provider of medical-grade physical activity and sleep monitoring solutions for the global scientific community. The company has FDA-cleared wearable activity monitors and robust data management and analytics platform to quantify human movement in academic and population health research.
As a strategic partner of ActiGraph, Techsol provides professional consulting services to implement Actigraph’s wearable technology solutions for pharmaceutical and life sciences organizations seeking to capture and monitor real-world physical activity, mobility, and sleep behavior for patients enrolled in clinical trials.


Our Consulting Services for Actigraph Products
We help biopharma and medical device companies to effectively use Actigraph’s wearables and other e-source technologies for real-time data capture during the conduct of clinical trials. Our team can provide strategic scientific and technology guidance on how ActiGraph solutions can be positioned in different types of clinical trials to facilitate real-time data capture for different types of patient assessments to improve study data quality.
We integrate the following ActiGraph’s technology platforms, data hubs and wearables to EDC, ePRO and other ancillary systems:
Centrepoint
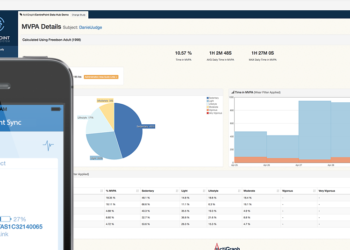
The CentrePoint technology ecosystem solves many of the practical and logistical challenges of objective activity and sleep monitoring within complex clinical trial environments and large scale, multi-site health research studies.
Read More
ActiLife
ActiLife is ActiGraph's premier actigraphy data analysis software is a powerful processing engine with an extensive selection of customer-driven features, analysis tools, and data management options to support a broad range of research objectives.
Read More
CentrePoint Insight Watch
The compact and stylish CentrePoint Insight Watch captures and records continuous high-resolution raw acceleration data to provide objective, real-world physical activity, mobility, and sleep measures, in near real time.
Read MoreSolution FAQs
ePRO (electronic patient-reported outcome) and eCOA (electronic clinical outcome assessment) have several advantages over traditional paper-based data collection methods. Here are some of the key advantages of using ePRO and eCOA:
-
-
Improved data quality: ePRO and eCOA can improve the accuracy and completeness of data by reducing errors in data entry, missing data, and illegible responses.
-
Increased patient engagement: Patients are more likely to participate in electronic data collection because it is often more convenient, accessible, and user-friendly. Additionally, ePRO and eCOA systems can provide real-time feedback to patients, which can encourage greater participation and engagement.
-
Enhanced data security: Electronic data collection methods can offer enhanced security measures that protect sensitive patient data, such as encryption, access controls, and audit trails. This can help prevent data breaches and ensure data privacy and compliance with regulatory requirements.
-
Time and cost savings: Electronic data collection can reduce the time and costs associated with paper-based data collection, including data entry, transportation, and storage.
-
Real-time data access: ePRO and eCOA systems allow researchers and clinicians to access data in real-time, which can support quicker decision-making and enable timely interventions to improve patient outcomes.
-
Improved efficiency: ePRO and eCOA systems can automate the data collection process, reducing the administrative burden on study staff and allowing them to focus on other important tasks.
-
Overall, ePRO and eCOA have many advantages that can help improve data quality, patient engagement, data security, and efficiency, while reducing costs and administrative burden.
We continuously provide support to sponsors for effectively using Actigraph’s solutions right from the beginning of the clinical study until closure by:
- Coordinating Site Shipping Logistics of Actigraph’s Wearables
- Delivering Training to Site Personnel on Product Usage
- Facilitating In-Trials Data Screening
- Monitoring Data Capture using different types of e-source technology
- Evaluating trial data and eliminate errors to ensure the integrity of information gathered using wearable devices
Following are the technology related services we offer for Actigraph’s solutions:
- Wearables and Device Implementation
- Integrate external APIs and Webhook applications with ActiGraph platforms
- Validate wearable functionality by testing them against study conditions
- Complete User Acceptance Testing prior to Study Start
- Prepare and Deliver Site and Participant Guides
- Near-real Compliance Monitoring
- Device Management and Ongoing Support during the Clinical Study
- Performing Data Cleaning and Transfers




