



Simplifying Clinical Investigation & Clinical Evaluation for Medical Devices
Understanding the Importance of Clinical Investigations
Clinical investigation is a systematic clinical trial conducted with human subjects to assess the safety and efficacy or performance of a medical device. However, the requirement of a clinical investigation varies depending on the risk-class of the medical device.
The need for clinical investigations depends on the ability of the existing data to adequately address the benefit/risk profile, claims, and side-effects in order to comply with the applicable Essential Requirements. Clinical investigations may therefore also be required for other devices, including for devices in class I and class IIa, and for class IIb devices that are not implantable.
Clinical Investigations – When are they Required?
Clinical investigation is a systematic clinical trial conducted with human subjects to assess the safety and efficacy or performance of a medical device. However, the requirement of a clinical investigation varies depending on the risk-class of the medical device.
The need for clinical investigations depends on the ability of the existing data to adequately address the benefit/risk profile, claims, and side-effects in order to comply with the applicable Essential Requirements. Clinical investigations may therefore also be required for other devices, including for devices in class I and class IIa, and for class IIb devices that are not implantable.
For most low- to medium-risk devices, a clinical investigation will not be necessary, as there is likely ample clinical data already available for collection and analysis to prove device safety and efficacy. According to MEDDEV 2.7.1, indications that your device may require clinical investigation include:
-
New design features and/or new materials
-
A new intended purpose
-
Making new claims about the device
-
New types of users
-
Serious direct and/or indirect risks
-
Invasiveness or contact with mucosal membranes
-
Increased duration of use or number of reapplications
-
Incorporation of medicinal substances
-
Use of animal tissues other than in contact with the skin
-
New recognized risks or the availability of alternatives with lower risks or higher benefits
What is a Clinical Evaluation and how is it related to Clinical Investigation?
Clinical evaluation is a process that is managed continuously throughout the product life cycle. It is an ongoing procedure to collect, appraise and analyze clinical data pertaining to a medical device and to analyze whether there is sufficient clinical evidence to confirm compliance with relevant essential requirements for safety and performance when using the device according to the manufacturer’s instructions for use. This ongoing process enables manufacturers to provide notified bodies and competent authorities with sufficient clinical evidence for demonstration of conformity of the device with the Essential Requirements throughout its lifetime (for example for CE marking, fulfillment of post-market surveillance and reporting requirements, or during surveillance procedures).
A key point to remember is that a clinical evaluation may lead to a clinical investigation if additional clinical data is needed to prove a device is safe and effective.
A clinical evaluation report shall be compiled to document the clinical evaluation and its output. The clinical evaluation report should contain sufficient information to be read and understood by an independent party (e.g. regulatory authority or notified body). Therefore, it should provide sufficient detail for understanding the search criteria adopted by the evaluators, data that are available, all assumptions made and all conclusions reached. The contents of the clinical evaluation report shall be cross-referenced to the relevant documents that support them. It should be clear which statements are substantiated by which data, and which reflect the conclusions or opinions of the evaluators. The report should include references to literature-based data and the titles and investigational codes (if relevant and available) of any clinical investigation reports, with cross-references to the location in the manufacturer’s technical documentation.
How We Can Help Across Different Studies
- Drafting the research question and study objectives (primary & secondary)
- Sample size calaculation and study population characterization
- Drafting study protocols and seeking necessary IRB / IEC approvals
- Begining subject recruitment and completing study procedures as per protocol
- Performing data cleaning and completing statistical analysis to present outcomes
- Always meeting timelines and avoiding delays

Services We Offer
Clinical Investigation
> Clinical Investigation Plan (Feasibility Studies & Pivotal Studies)
> Study Management (Pre-study Planning to LPPV)
> Clinical Data Management & Medical Coding
> Biostatistics & SAS Programming
> Study Reports Compilation & Data Submission

Clinical Evaluation
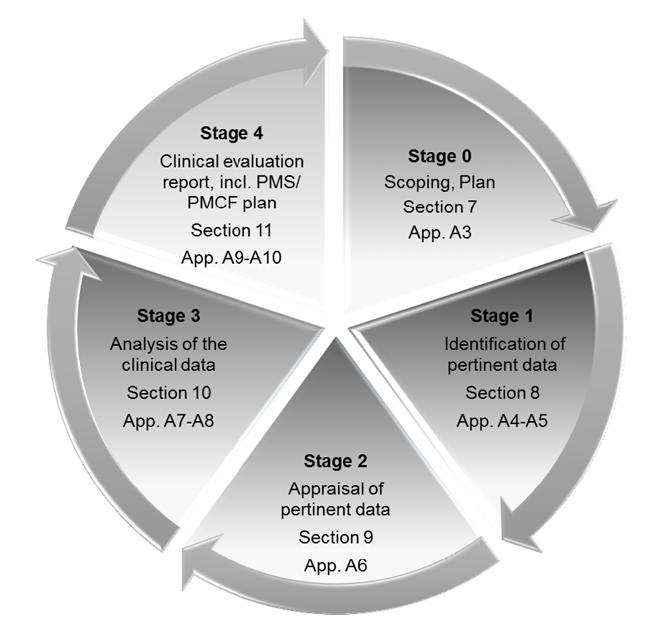
The clinical evaluation is based on a comprehensive analysis of available pre- and post-market clinical data relevant to the intended purpose of the device in question, including clinical performance data and clinical safety data. Our team can support in performing clinical evaluations by:
> Drafting scope, objectives and plan based on the nature of the device
> Analyzing all available data generated through clinical investigation, audits, safety reporting, etc. (Study reports, PMS & FSCA reports, customer complaints, trends, etc.) and data retrieved from literature pertinent to the device
> Drafting and executing the appraisal plan to determine product safety, quality and performance
> Completing clinical data analysis with reference to qualitative and quantitative measures
> Preparing / updating the CER Report and drafting recommendations for Post-market clinical follow-up.



