



GMP Compliance Services
GMP regulations mandate a risk-based quality approach to manufacturing, enabling companies to minimize or eliminate instances of contamination, mixups, and errors. Key GMP processes include including record keeping, personnel qualifications, sanitation, cleanliness, equipment verification, process validation, and complaint handling. Failure of firms to comply with GMP regulations can result in very serious consequences including product recall, plant closure, penalties, fines and severe legal proceedings.
As most GMP requirements are very general and open-ended, manufacturers find it difficult to interpret critical requirements and implement necessary quality and risk controls that assure proper design, monitoring, and control of manufacturing processes and facilities. The Regulatory team at Techsol specializes in providing very focussed cGMP regulatory services that meets the quality criteria mandated by agencies.
With over 15+ years experience, we carry out various audits including full Site audits which focus on the facility’s quality management systems and cover a large number of products (API and Intermediates) with the following principles:
- Ensuring accuracy and completeness of information
- Drafting content as per latest regulatory guidelines
- Effectively communicating complex information
- Applying latest methods in scientific research
- Leveraging latest technology for content creation
- Always meeting timelines and avoiding delays

How We Can Help
At Techsol, our expert team of Medical writers have an extensive research background, scientific knowledge, and ample experience in contributing to clinical development programs for global pharma companies.
Following are the key focus areas where our medical writing team has the expertise to deliver high-quality services throughout the drug / device development lifecycle as per applicable regulatory guidelines:
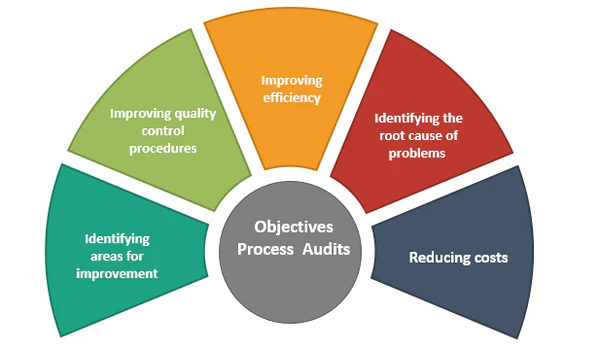
GMP Process Improvement Audits
Clients trust our expertise to complete the following types of audits as part of the cGMP compliance gap assessment exercise.
QMS Audit: Review GMP compliance and process efficiency
Root Cause Audit: Investigating process failures and associated CAPAs
GAP Audit: Checking GMP Systems, Infrastructure, Resources & Processes
Vendor Audit: Vendor Qualification or Re-qualification Audit
Pre-inspection GAP/Mock Audit: For proactive agency audit preparation
Sponsored Audits: Audit done on behalf of Sponsor

GMP Training
We provide in-depth training for manufacturing teams, so you can avoid costly regulatory findings from global agencies. Following are the training focus areas that our team can deliver:
> 21 CFR cGMP Regulations and associated QMS Processes
> SOPs, Checklists and Work Instructions preparation and operating plans
> Facility, Equipment, Intrument, Sanitation, Cleaning validation processes
Access Key cGMP Regulations
United States
Food and Drug Administration GMP Regulations
21 CFR Part 4 – Current Good Manufacturing Practice Requirements for Combination Products (As of 1 April 2013)
21 CFR Part 210 – Current Good Manufacturing Practice in Manufacturing, Processing, Packing, or Holding of Drugs (As of 1 April 2013)
21 CFR Part 211 – Current Good Manufacturing Practice For Finished Pharmaceuticals– (As of 1 April 2013)
Historical preambles announcing changes and comments regarding 21 CFR Parts 210 and 211.
21 CFR Part 212 Current Good Manufacturing Practice for Positron Emission Tomography Drugs – (As of 1 April 2013)
21CFR Part 110 – Current Good Manufacturing Practice in Manufacturing, Packing, or Holding Human Food (As of 1 April 2013)
Historical preambles announcing changes and comments regarding 21 CFR Part 110
21 CFR Part 606 – Current Good Manufacturing Practice For Blood and Blood Components (As of 1 April 2013)
Historical preambles announcing changes and comments regarding 21 CFR Part 606
21 CFR Part 820 – Quality System Regulation (As of 1 April 2013)
Historical preambles announcing changes and comments regarding 21 CFR Part 820
21 CFR Part 111 – Current Good Manufacturing Practice in Manufacturing, Packaging, Labeling, or Holding Operations for Dietary Supplements (As of 1 April 2013)
Historical preambles announcing changes and comments regarding 21 CFR Part 111




