

Integrated Drug Safety and Signal Management Solution For Biopharma and CROs
Safety Systems and information technology is an integral part of setting-up and running effective Pharmacovigilance operations for all pharmaceutical and biotech companies, regardless of their size. Our strategic consulting services coupled with our industry leading safety technology solutions help clients to optmize their drug safety operations.
Introducing AccelSafety
AccelSafety’ is a comprehensive pharmacovigilance platform of Techsol built around the Oracle Argus Safety application to address business needs of unified collection, medical assessment and regulatory reporting of clinical & post-marketing safety surveillance data.
Our fully-managed cloud solution facilitates emerging biopharma, device manufacturers and service providers to manage safety data and take informed business decisions in alignment to global compliance requirements.

Key Solution Benefits
- Fast-track Argus Safety Implementation, Migration and Validation with 70% effort reduction
- Use our expertise to migrate your legacy safety data to AccelSafety Cloud in a secure manner
- Establish simplified lean case processing through customized configurations and native automation
- Gain comprehensive operational insights through our advanced Reports, Line listings and Analytics package
- Leverage our 24x7 Argus Safety Service Desk to address user requests, incidents and change management
We have been a trusted Oracle partner for the last 12+ years and have successfully delivered over 150+ projects across 5 continents.

With AccelSafety, clients can choose the most suitable solution model which can meet their business needs.
AccelSafety Standard
For core Drug Safety operations and e-submissions
AccelSafety Enterprise
Standard plus Advanced Analytics and Datamart options
AccelSafety Premium
Includes Enterprise plus Oracle Empirica Signal & Topics
AccelSafety Japan
Core Module with Add-on for integrated Japanese operations
AccelSafety Project Services
With 12+ years of experience, our Safety Technology experts have worked with emerging/ SMB, generic, innovators, large global pharma and CRO companies to establish and optimize their drug safety operations. We can deliver the following value-add services as part of the ‘AccelSafety’ cloud implementation for your organization.

Plan your long-term success by establishing and optimizing your Drug Safety Systems and Processes with our unparalleled subject matter expertise.
Safety Management Process Definition
As part of the AccelSafety solution implementation, our subject matter experts can provide knowledge support to:
> Determine the right Argus Safety solution model that fits your organization’s business needs
> Setup the core business processes for safety operations such as ICSR Case Processing, Regulatory Reporting and Safety Signal Management
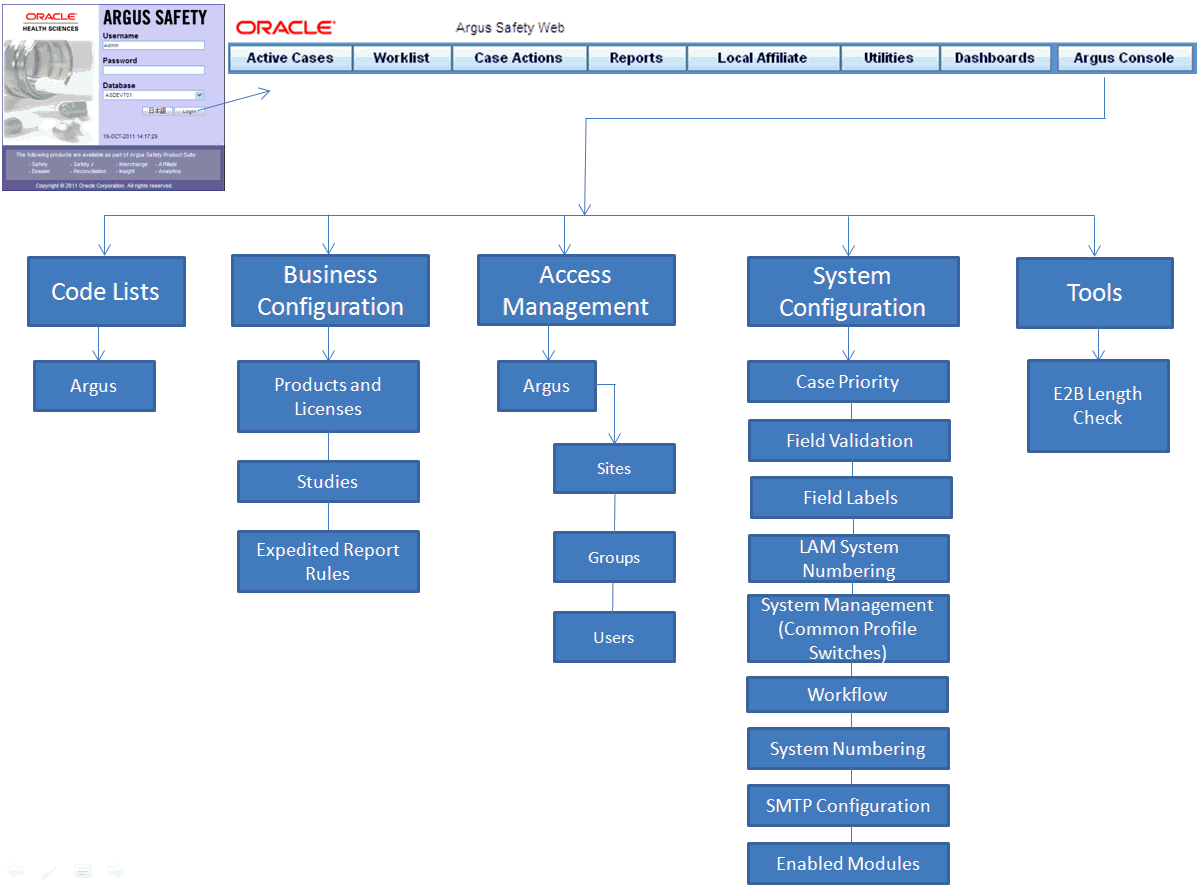
Argus Safety Configurations Setup
Our Argus certified SMEs offer extensive technical and functional guidance to configure:
- Products, Studies and associated workflows
- Auto-narratives and letter generation
- Global Reporting Rules
- Optimized Business Rules for lean workflows
- Dictionary Management (MedDRA English & Japan, WHO Drug)
- Users & Roles Administration
- Query & Reporting Services
- Signal Management

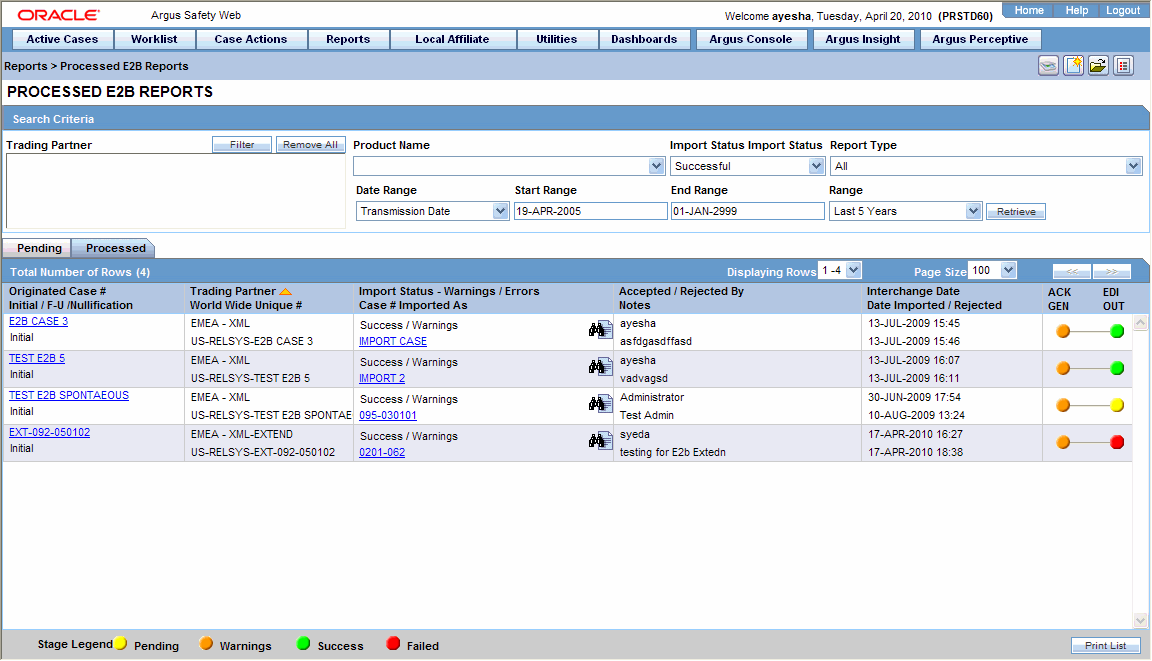
Setup Regulatory Reports and Configurations
Based on your global compliance requirements, we can setup and automate the schedule for generating the following regulatory related reports:
- ICH: E2B R3 electronic data submission
- ICH: E2C periodic safety update report (PSUR)
- ICH clinical trial periodic report and EUSAR
- IND and NDA periodic report
- CIOMS II line listing and CIOMS V
- EMEA/CPMP reporting
Electronic Submission Gateway Setup
As a trusted partner of Oracle and Axway, we can enable the complete automation of your electronic submissions of safety case data information to Regulatory agencies and your business partners. We have tailored process based on our working experience in setting up and pilot testing with below Regulatory agencies:
- FDA, EMA ( and the associated NCA)
- PMDA
- Health Canada
- MHRA

> With AccelSafety, our team can complete fast-track Argus Safety implementation, data migration and validation with 70% effort reduction
Safety Sytem Implementation, Migration & Validation
The AccelSafety platform is hosted on a regulatory GxP compliant cloud. All the Argus application feature functionalities are validated in a comprehensive manner. Clients will be provided with the latest IQ/OQ documentation to account for 21 CFR Part 11 compliance.
Business users can quickly execute UAT after undergoing a hands-on training delivered by our Argus Safety Experts.
Solution FAQs
Techsol’s AccelSafety solution is delivered on a validated, GxP compliant, fully-managed cloud platform that is hosted on Amazon Web Services (AWS). It has security and quality certifications such as ISO 9001, ISO 27001, ISO 27017 and ISO 27018 that accounts for information security.
All ongoing systems validation and preventive maintenance of Oracle Argus Safety and associated add-on applications will be managed by Techsol throughout the business lifecycle.
- Techsol owns the responsibility for the end-to-end management of Argus Safety application
- Burden of validation & compliance management is significantly reduced
- Security option with Single sign-on is available
- Includes all future application version upgrades, dictionary upgrades, and security updates
- Considerably reduces Total Cost of Ownership (TCO)
With the use of advanced technology and quality-driven processes, our medical writing team members collaborate with Biostatistics, Medical Affairs, Drug Safety and Clinical Data Management teams to deliver accurate and purposeful publications and other medical content with a quick turnaround with 100% compliance to global regulatory requirements. Every medical writing project is meticulously managed and delivered with stringent scientific, statistical, editorial and quality control review.




