Engage With Us to Win Exciting Prizes
As the platinum sponsor of WDS 2022, Techsol Life Sciences is looking forward to meeting you and your team at this years’ event. Stop by our booth to have a conversation about your business needs and present our solutions. Also, don’t miss your chance to participate in our ‘Spin & Win’ contest to take away the below exciting prizes.





Day – 1 (October 4th, 2022)
We are proud to announce that Satya Sagi, Founder & CEO of Techsol Life Sciences will be delivering an insightful presentation on the “Transformation of pharmacovigilance operations to enable PV Ops Oversight through Digitalization” at 2:40 PM to 3:00 PM.
Key takeaways:
Get introduced to concept of Pharmacovigilance Oversight
Learn about how to address current PV Operational Challenges
Formulate a roadmap for PV process digitalization, and
Learn about the long-term benefits after PV Ops Digitalization & Oversight implementation.

During the conference session breaks, you are welcome to attend the following presentations at the Techsol booth.
- Digitalize and Automate Literature Screening while increasing efficiency and achieving compliance (Timing : 10:40 to 10:50 , 4th Oct 22)
- Streamline Drug Safety Aggregate Reporting Through Digitalization (Timing : 13:00 to 13:10 , 4th Oct 22)
- AccelSafety – Integrated Drug Safety and Signal Management Solution , Powered By : Oracle Argus (Timing : 13:30 to 13:40 , 4th Oct 22)
- The Future of GxP Operations Oversight & Compliance Governance (Timing : 10:40 to 10:50, 5th-Oct-22 @ Booth 3)
- Leverage the Power of Digital Safety Data Exchange Agreements and Streamline Partner Oversight (Timing : 13:00 to 13:00, 5th-Oct-22 @ Booth 3)
- AccelSafety – Integrated Drug Safety and Signal Management Solution , Powered By : Oracle Argus (Timing : 13:30 to 13:40, 4th-Oct-22 @ Booth 3)
Techsol’s WDS 2022 – Lucky Draw Contest
Don’t miss the fun while you can get lucky this WDS 2022!
We welcome you to interact with our team, fill in your entry and drop your Business card to participate in our two-day lucky draw contest to win a Marshall Speaker and Gift Cards.

Day – 2 (October 5th, 2022)
SDEA Lifecycle Management involves collaborative effort and timely communication from multiple stakeholders while drafting agreements for various PV Processes. Don’t miss this insightful panel discussion with thought leaders from the industry to streamline agreements, exchange and reconciliation.
Topic: Key Opportunities to Increase Efficiency and Achieve Global Compliance Across SDEA Operations While Engaging With Partners & Affiliates (See Panelists below)
- Ram Vempati, Head of Pharmacovigilance, Arcellx Inc.
- Victoria Bartasek, Senior Associate Director, Global Pharmacovigilance, Boehringer Ingelheim Pharma GmbH & Co. KG
- Paridhi Anand, Associate Director, PV Operations, ICSR Quality and Analytics, Moderna Therapeutics
- Satya Sagi, Founder & Chief Executive Officer, Techsol Life Sciences

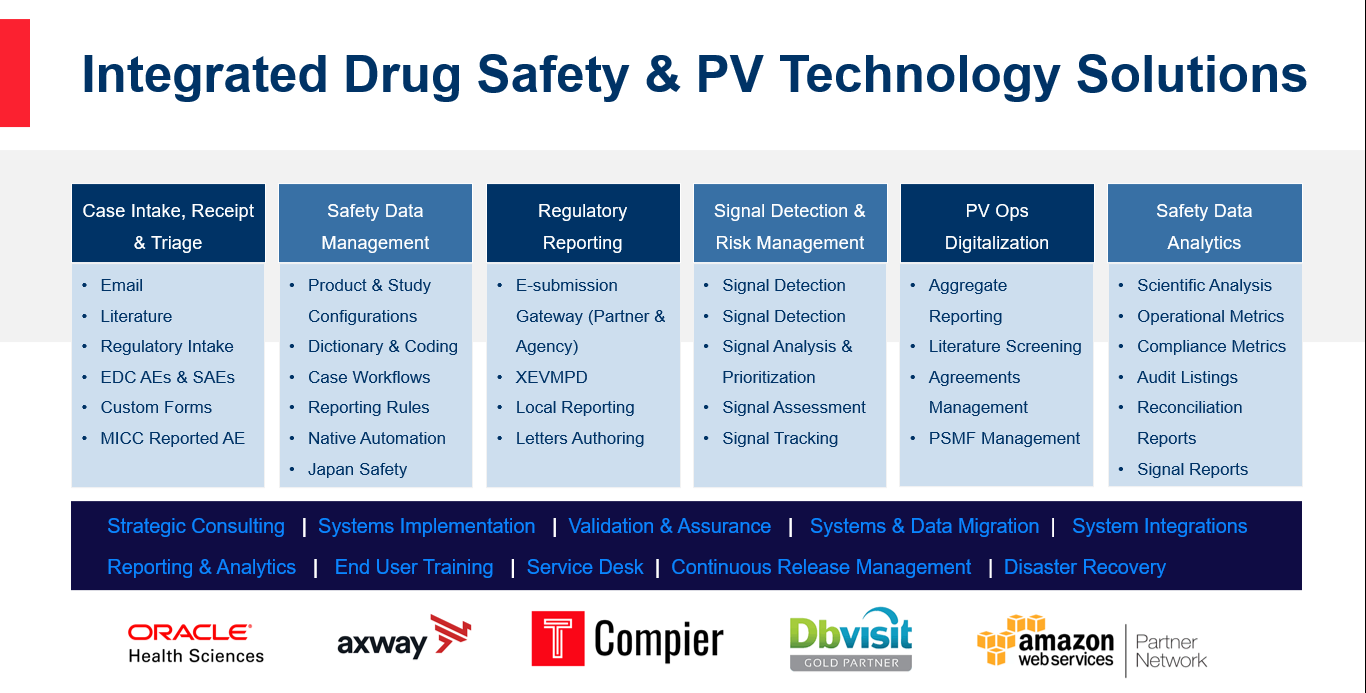
Learn about our end-to-end, integrated Drug Safety & Pharmacovigilance Technology Solutions and Functional Services that we delivered for more than 80+ clients. Our strategic solution delivery model includes a combination of Oracle Argus Safety, Compier PV Ops platform and SciMax medical affairs platform. Have a chat with our team and send us your requirements and business priorities. We’d be happy to present our unified business solutions that increases quality, compliance, and overall value.