- December 3, 2021
- Posted by: Techsol Life Sciences
- Category: Pharmacovigilance

Pharmacovigilance involves the collection, detection, assessment, and monitoring of data on Adverse Drug Reactions (ADRs) which must be evaluated to derive vital safety information. Signal detection involves monitoring the adverse reaction data for patterns that suggest new safety information. Safety signals can be detected from an extensive range of sources, such as spontaneous reports, clinical studies, and scientific literature.
The EudraVigiliance database is an important source of information on suspected adverse reactions and signals. Commission Implementing Regulation (EU) No 520/2012 (article 18) requires EMA, national competent authorities (NCAs), and marketing authorization holders (MAHs) to continuously monitor the data available in EudraVigiliance. It also requires MAHs to inform EMA and national competent authorities of validated signals detected when monitoring the database. [3] The evaluation of safety signals is essential to ensure that regulatory authorities have the latest information on the holistic benefits and risks of a medicine.
What Is A Signal?
The definition of a signal as provided by the council for international organizations of medical sciences (CIOMS) Working Group 8 is: “Information that arises from one or multiple sources (including observations and experiments), which suggests a new potentially causal association, or a new aspect of a known association, between an intervention and an event or set of related events, either adverse or beneficial, that is judged to be of sufficient likelihood to justify verificatory action”. [1]
Contact us by submitting a business inquiry online. We will get back to you very soon.
In essence, a signal is a hypothesis of a risk associated with medicine along with supporting data. The evidence in a signal is an early indication and not definitive as it substantially changes as the data accumulates over time. The evaluation of safety signals should be a continuous process in pharmacovigilance as it is imperative for manufacturing authorization holders to have a well-defined process to capture, evaluate and take immediate action on potential PV risks as needed. A signal may be generated either by quantitative analysis through data mining algorithms (DMAs) and statistical analysis or by qualitative analysis of spontaneous reports.
A signal may provide supplementary or new information about adverse or beneficial effects of an intervention, or data about a known association of medicine with an adverse drug effect. For example: postulating a mechanism based on the range of severity of effect, suggesting a dose range, and indicating an at-risk group or sometimes lack of effect by a particular medicine.
What Is Signal Management in Pharmacovigilance?
The process of signal management in pharmacovigilance consists of a set of activities that aim to determine if there are new risks associated with an active substance or a medicinal product or whether known risks have changed based on a thorough analysis of individual case safety reports (ICSRs), aggregate data from active surveillance systems or scientific literature. The set of activities includes the following steps:
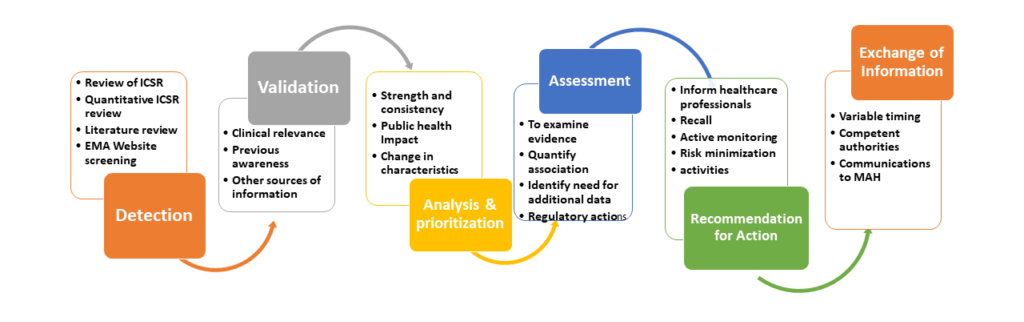
The sources for signal detection can come from:
- Spontaneous reporting
- Active monitoring systems
- Interventional studies (clinical trials)
- Non-interventional studies (Pharmacoepidemiologic studies)
- Non-clinical studies (e.g. Animal toxicology studies)
- Systematic reviews (i.e. Review of the published literature)
Signal Management Overview
A signal must be assessed to determine its validity by investigating the clinical relevance and by comparing it with the standard available information. The main areas to focus on an adverse event and trends are:
-
- Case Data and Case Quality:
- Is the signal associated with a trend related to frequency, seasonality, or geographical location?
- What are the expectations based on product distribution or clinical data as compared to historical data?
- Is the data of good quality or most of the cases assessed as unclassifiable because the information provided is insufficient or contradictory?
- Patient Data:
- Is the signal associated with a trend related to the patient’s age, sex, profession, lifestyle, medical conditions, or other relevant factors?
- Product Data:
- Is the signal associated with a trend related to the product’s use (or misuse), serial/batch, location of the manufacturer, labeling, distribution?
- Event Data:
- Is the signal associated with a trend related to severity, frequency, time of onset, duration, progression, the outcome of the event?
- Case Data and Case Quality:
Once a signal is identified, it must be further evaluated and classified either as a new risk or a previously reported risk. It is conducive to document and store all identified, ongoing, or closed signals as the information will be readily available to the signal management team for easy assessment and decision making. Do not discard refuted signal data, maintaining this in a searchable document management system can be very helpful for responding to National Competent Authorities (NCAs) inquiries or in evaluating similar signals in the future.
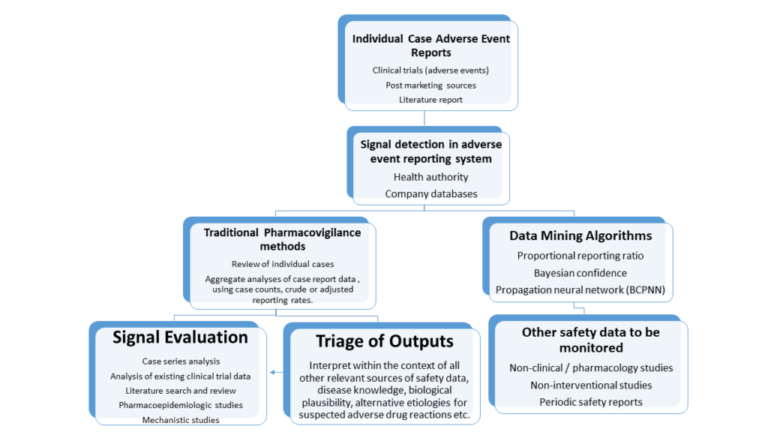
Challenges in Signal Management
Throughout the signal management process, multiple challenges can be encountered such as:
- Continuous increase in data volumes
- Large numbers of potential safety signals
- Drug-event combinations identified through disproportionality analysis
- Large number of false positives
- Signal scores below the threshold limit
- No new cases or changes recorded over time
- Associated with labeled or other reported event(s)
- Reducing false positives to minimize the workload for signal evaluators
- Providing better safety information before clinical trials begin
Best Practices for Signal Management
The best practices mentioned below refer to general areas to specific steps in the signal management process.
Comprehensive access to data is essential to perform signal management activities
Access to all valid documents and data sources is essential to PV evaluators involved in signal management to ensure thorough assessment. [2] Some of the most relevant documents and data sources are clinical trial data, product application dossier, SmPC, patient leaflet, PSURs, RMPs, EPITT, decision-making documents (e.g. from scientific committees or regulatory procedures), scientific literature, data provided by a marketing authorization holder (MAH) and data sources outside spontaneous reports, such as registries and databases, etc.[2]
Disproportionality methods should be applied to databases of the right size and background.
The practice of disproportionality methods is not suitable in all situations as the application of disproportionality methods to a dataset with a limited set of drugs or events reported may not provide any added benefits compared to using qualitative methods. Hence, it may be more appropriate to apply qualitative methods (e.g. count of case reports) for small databases.
The method of disproportionality analysis is insignificant compared to the chosen threshold for detection
It is important that any signal threshold applied is appropriate to the size of the database, the products it contains, and the level of sensitivity. This applies when complex disproportionality methods or rule-based approaches (such as reviewing all fatal case reports) are employed. [2] It is imperative that the chosen approach can be shown to identify signals through a validation process (the statistical analyses should be robust against false associations due to random variability). This process should ensure that the approach can detect issues that have been identified in the past for the products under review.
Prioritization should be a continuous process
Signal prioritization should be a continuous process. A standardized approach should be in place with predefined criteria and validated constantly. Careful prioritization of signals and related variables need to be implemented as a continuous process for effective signal management. [2]
Future Trends in Signal Management
Automated signal management processes will likely be the future trend as it augments quality and efficiency while remaining compliant. The computerized algorithms used in automated processes are able to complete many of the arduous steps, such as signal evaluation and validation, involving activities at the case and AE level and significantly reduce time, resources and expenses for signal management.
Using AI such as neural networks enables neural signal detection for greater modeling power and flexibility than the non-AI-based methods, greatly improving detection accuracy. Another method called multimodal signal detection represents an advanced process that can be employed when searching through large volumes of data located in disparate data sets. In this process, signal statistics are aggregated across the available data sets in order to generate a more accurate composite signal score.
Sponsors need a reliable and centralized system to track signals, ensuring all safety actions are reconciled in a standard manner, independent of therapeutic category or geography. The risk associated with a siloed and inefficient PV infrastructure is enormous. Companies need to scrutinize their safety signal information systems to minimize patient safety risks and also to avoid audit findings.
How We Can Help
Techsol has deep expertise across all signal management activities, including implementation, detection, validation, and assessment by using a wide range of sources, such as spontaneous cases, safety data from clinical trials, and literature reports. We are also experienced in the EudraVigilance Data Analysis System (EVDAS) functionality and can ensure that sponsors and Marketing Authorization Holders (MAHs) thoroughly comply with their signal detection and management obligations. With our technology and expertise organizations can benefit from an end-to-end Signal Management solution according to industry best practices and recent regulations (CIOMS VIII, GVP Module IX). To know more, please reach out to us at info@techsollifesciences.com.
References:
-
- CIOMS. (2010). https://cioms.ch/wp-content/uploads/2018/03/WG8-Signal-Detection.pdf (No. WG8-signal).
- SCOPE Work Package 5 Signal Management. Best Practice Guide – PDF Free Download. (2016). https://docplayer.net/79618432-Scope-work-package-5-signal-management-best-practice-guide.html
- EMA. (2021, September 16). Signal management. European Medicines Agency. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/signal-management



