-
Understanding the Importance of CMC (Chemistry, Manufacturing, and Controls) documents in the Pharmaceutical Product Lifecycle
April 8, 2024 -
MoCRA Made Easy: A Comprehensive Guide to Cosmetic Safety Compliance
January 29, 2024 -
Emerging Trends in Regulatory Affairs by Adapting to Latest Technological Advancements
July 5, 2021 -
Global Regulatory Changes in Biopharma
June 21, 2021 -
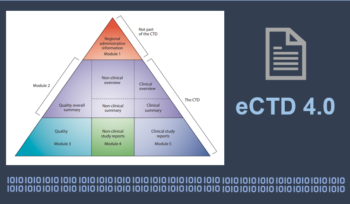
ICH’s eCTD Version 4.0 – Objectives, Major Updates, Resulting Advantages and Possible Challenges
June 14, 2021
How can we help you?
Contact us by submitting a business inquiry online. We will get back to you very soon.