- July 5, 2021
- Posted by: Techsol Life Sciences
- Category: Regulatory Affairs

Globally, Life Sciences and Pharmaceutical industry continues to adopt advanced technology and novel therapies to improve quality of medicines, medical devices and biologics. But legacy guidelines do not offer enough regulation over these evolving ways of triage and treatment. To ensure the high quality medicines and diagnostics are available for patients, regulatory agencies identify and assign new policies or revise existing guidelines to review the novel therapeutics and devices amicably and thus establish their safety and efficacy.
The breakout of a global pandemic has had a similar impact on the global regulatory bodies as they try to quickly review and approve safe products to counter COVID-19. Operating drug development process, pre-marketing and post-marketing clinical studies, manufacturing, handling their supply chain and distribution in the new business environment needs an alternative view point for regulatory bodies. They are considering various emerging roadblocks for the industry, and devising and issuing guidelines corresponding to those processes.
As health authorities issue new policies and guidelines, organizations might have to re-evaluate their regulatory affairs structuring and functionality, and modify them to operate as per new regulations that come into place. En route, they could come across multiple challenges at various phases in the process. What new trends are awaiting to be explored on the horizon and how would they affect the conduct of regulatory affairs for the stakeholders? Grasp the details in our deep dive about ‘Emerging Trends in Regulatory Affairs by Adapting to Latest Technological Advancements.’
Contact us by submitting a business inquiry online. We will get back to you very soon.
Current Challenges in Regulatory Affairs for Sponsors
As Pharma industry continues to make strides into offering better quality of medicines, sponsors are still coming across challenges in both process and technology adoption.
Process Challenges:
- Obtaining regulatory approval for a drug is often time-consuming especially during the pandemic, which leads to higher costs, a more complex supply chain, and a requirement for refined systems to sustain regulatory compliance
- Understanding and staying abreast of evolving regulatory requirements
- Collaboration with multiple stakeholders has become elusive as COVID-19 has restricted movement and digital connectivity is not uniformly resulting in communication gaps
- Submission of adequate evidence by the sponsor from in vitro studies to confirm that the proposed clinical trial is reasonably safe
- The marketing authorization application (MAA) must contain evidence of the drug’s chemistry, manufacturing, and controls (CMC) (details of product manufacturing, product stability, and shelf-life), and preclinical studies of pharmacology, pharmacokinetics (PK), and ADME
- Developing and maintaining country-specific versions of the same information in order to meet regional health authorities requirements
- Maintaining different processes for manufacturing the same product to ensure availability of product, which increases inventory segmentation and potential for errors in terms of producing and regulatory compliance
- Managing GxP inspections that are required for approval of submissions
Technical challenges:
- Adapting to modern technology that is dynamically changing is laden with constant updates and usage of multiple electronics/software tools at times making the process tough
- As novel approaches and techniques come to light, current study designs may not be compatible to determine the efficacy of the drug
- As medical research keeps evolving, inventing new techniques and approaches are often a prerequisite to scrutinizing new medical research
New Trends in Regulatory Affairs to Expedite Review Process
Pharmaceutical companies have a deep-rooted process for clinical development, but the outbreak of covid-19 pandemic has massively disrupted this traditional way of testing new drugs. As pandemic unravels limiting the scope of clinical studies as we know, drug manufacturing companies will need to continue to expand their search for other possibilities and devise new ways of working with accelerated digital adoption and innovation. Pharma companies can integrate the following approaches and redefine clinical development.
Adopting Real World Evidence (RWE)
Over the past few years, manufacturers are progressively using real-world evidence (RWE) to support clinical trial designs and observational studies to create innovative, new treatment approaches. In 2017, the FDA relied on RWE to expand the use of Edwards Life sciences’ transcatheter aortic valve replacement device for valve-in-valve procedures, the first FDA approval based on RWE without requiring new clinical trial data. COVID-19 pandemic has fast-tracked this trend towards increasing RWE as a way to test new treatments in a few weeks or months rather than the prolonged timeline typical for randomised controlled trials (RCTs).
Adopting Virtual First Approaches
In response to the COVID-19 emergency, many organizations are now pursuing virtual trials, especially where interventions are well understood and seen as low-risk (e.g., new indications of existing therapies for chronic diseases). The US FDA and the EMA have issued guidance in support of virtual trials. The main benefit of conducting virtual trials is that it allows to recruit specific patient segments quickly irrespective of where they reside, thus offering flexibility of location. The industry experts suggest that the virtual trials are more time-efficient, as it takes just 4 months to recruit patients unlike 7 months in the traditional process.
Adopting Modern Technology
The use of computers, mobile devices, and other wearable biosensors gathers and stores a large volume of health-related data. This data holds the potential to permit us to enhance the design and conduct clinical trials and studies within the health care setting. Additionally, with the advancement of sophisticated, new analytical capabilities, we are now ready to analyse these data and apply the results of our analyses to medical development and expedite the approval process.
Using Real-World Data (RWD)
Real-world data (RWD) captures the patient health status which can be routinely collected from a range of sources, such as:
- Electronic health records (EHRs)
- Claims and billing activities
- Product and disease registries
- Patient-generated data
- Data gathered from even mobile devices.
In 2016, the FDA focused on considering real-world evidence to alleviate the off-label drug usage in approvals. So, when the FDA reviews and approves a selected drug, it’s inspecting clinical data of controlled clinical studies as the patients are in a controlled environment and can be continuously monitored and carry out direct comparisons, usually between either placebos during treatment or between different treatments.
But once we start dosing patients in the real world, there’s plenty of variation in the data that gets collected, because we don’t know what patients’ daily life activity and resulting exposure. Thus, RWD helps in monitoring post-market safety, adverse events and to make judicious regulatory decisions.
Telemedicine
Originally developed to aid healthcare settings, telemedicine involves the use of electronic communications to provide clinical services to patients without an in-person visit. Telemedicine technology is often used for follow-up visits, management of chronic conditions, medication management, specialist consultation and a host of other clinical services that can be provided remotely via secure video and audio connections. But telemedicine applications can be augmented to be used to effectively manage clinical trials and gather adverse event data.
Telemedicine apps also can be linked with biosensors to gather and monitor data. It offers scope for clinical investigators to continuously monitor their subjects remotely without having to constrain them to a healthcare facility. By limiting the subjects’ movement, their exposure to external agents is curtailed offering improved study conditions. As subjects need not travel, their appointments and follow-ups are effective and regular. The slew of benefits noticed provide both revenue and time saving advantages. The onus lies on study designers to plan and execute them.
Pragmatic Clinical Trials (PCTs)
The delay in obtaining evidence from clinical trials for COVID-19 treatment prompted a new and improved approach in conducting a randomised clinical trial. According to a study, by optimizing the current research ecosystem and accelerating regulatory approval for pragmatic (practical) clinical trials (PCTs), timely evidence generation can be accomplished to better guide drug approvals particularly at the times of pandemics.
PCTs are defined as patient-centred, outcome-based trials examining the comparative benefits and risks of therapeutic interventions to inform clinical and/or policy decision-making. These trials often employ electronic health records (EHR)-embedded research designs to rapidly test important clinical questions.
PCTs, measure the effectiveness of an intervention in a real-world environment. There are typically few to no exclusion criteria in the population of interest and the intervention is provided along with routine care by a heterogeneous group of clinicians. While designing the study, external validity is valued to maximize the generalizability of the study.
PCTs are more viable today given the proliferation of collaborative research networks and integrated health systems in hospitals, clinics, with unified EHR.
Advantages of Pragmatic Clinical Trials
- PCTs utilize EHR-based strategies for automated screening and randomization, simplify consent processes, and incorporate follow-up assessments into routine clinic visits.
- Equipped with machine learning and advanced language processing tools, endpoint ascertainment can be achieved through automated periodic EHR review allowing interim analysis and safety monitoring.
- Ease of data capture makes PCTs better suited to adaptive trial design that allows modification of tested interventions and sample size calculation.
- Testing in a real-world set-up leads to more generalizable results which can be implemented in clinical practice.
- The reduced per-patient cost allows large sample sizes in PCTs, which mitigates most protocol deviations.
Moreover, contrary to traditional RCTs, PCTs with large sample sizes can assess multiple interventions and outcomes including patient-centred outcomes (e.g., quality of life), health service delivery metrics (efficiency and cost-effectiveness).
Big Data Analytics
Big data is a collection of a large volume of data that is generated in hospitals and clinics on a day to day basis with increased velocity and variety. Over a period of time, there has been another “V” that gained momentum – Veracity, which refers to the quality of the data.
Big data can analyse and streamline complex business processes that lead to better decisions and strategic business moves. It has a profound influence on the pharma and life sciences industry.
- Clinical trials – Big data can help in recruiting patients for clinical trials by tracking the genetic information and disease status of subjects most relevant to the study.
- Drug discovery – It can support researchers in predictive modelling for drug discovery.
- Precision medicine – It can enhance the diagnosis and treatment of several disorders by capturing and understanding a patient’s genetic make-up, environmental factors and behavioural patterns.
- Research and Development – Leveraging big data in the pharmaceutical industry will aid businesses in gaining a holistic awareness of the variety of drugs and their development.
Benefits of Big Data in Pharma
Big data has a range of applications thus offering multiple benefits. From among them, the important ones are listed here.
- Capturing real-world patient data
- Better disease understanding
- Better design of clinical trials, products and treatments
- Reduced cost, enhanced quality and efficiency
- Customized drug development
- Prediction of disease trends and medical forecasting
- Detection of severe adverse events
- Comparative effectiveness of drugs
Artificial Intelligence
Artificial Intelligence is an inclusive term used to define the use of machine learning, deep learning and Natural Language Processing (NLP) to simulate the human capability of analyzing and comprehending complex medical data.
Compared to traditional analytics and clinical decision-making techniques, AI allows better performance. Continuously learning algorithms interact with training data, allowing humans to gain unprecedented insights into diagnostics, care processes, treatment variability, and patient outcomes. They have the ability to improve the quality of data resulting in producing precise and accurate outcomes.
The Pharma industry is significantly gaining from AI-driven efficiency improvements in R&D, manufacturing, sales and marketing.
Benefits of Artificial Intelligence
- AI deep learning algorithms help in quick and precise diagnosis of serious diseases that could lead to more timely therapeutic intervention with better-targeted medicines.
- Ancillary benefits for technology companies active in pharmaceuticals, as algorithms for target identification, clinical-trial recruitment become more ingrained R&D processes.
- AI provides increased patient access to care.
- AI facilitates improved patient screening and records management
- AI is heading towards actualizing “virtual biopsies” and advance the innovative field of radiomics. They harness image-based algorithms to characterize the phenotypes and genetic properties of tumours.
- AI reduces the burden of electronic health record (EHR) use by automating the routine process of clinical documentation.
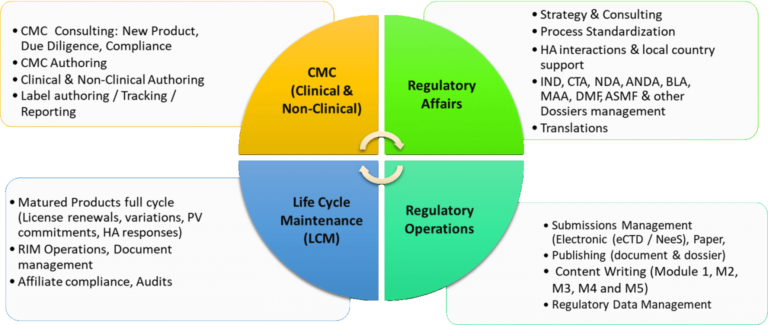
How Techsol’s rCoE (Regulatory centre of excellence) ensures adoption of industry best practices in the client regulatory affairs department?
Techsol’s Regulatory Center of Excellence (rCOE) strives to keep all our clients updated on the latest trends and changes across different types of GxP and Data Privacy regulations. We evaluate business process risks and put forward ideal best practices for adapting and implementing enterprise-wide compliance actions. With extensive GxP regulatory experience, Techsol has been helping several global companies to strategize and evolve continuously from a compliance perspective right from development to commercialization.
We have a wide range of capabilities around the following services:
- CTD/eCTD Submission
- Submissions of CTAs
- Review and compilation of Product
- Registration dossiers (NDA/MAA/ANDA, IND/CTA, DMF)
- Life Cycle Management of Annual Reports, Variations, Renewals, Safety Reports
- 510 (k) Submissions
- PMA Submission
- Preparation and review of T-License, Import/Export dossiers
- Response to regulatory authorities or review of deficiency responses
- Submission of eCTDs for IND/CTA, NDA/MAA, ANDA
- Regulatory Agency Liaison
- DMF compilation for Submission to EU and USFDA in CTD format
- GMP compliance services for APIs and finished manufacturing facilities
- AI model development and platform services (ML, NLP,RPA)
- Big Data Management
Our specialised Big Data analytics team helps build predictive models for drug discovery and engineer real-time visualization of data streams. Predictive modelling enables you to predict drug interactions, toxicity, and inhibition and thus expedites the whole process of drug discovery.



