- March 3, 2021
- Posted by: Techsol Life Sciences
- Category: MedTech

Today, more and more combination products are being developed to address the ever-increasing demands of patients who require more advanced and more effective treatments.
Earlier, traditional pharmaceuticals, medical devices, and biologics were the only options for treating the various medical conditions that people experienced. However, the steep rise in aging populations, has caused a shift in the healthcare industry. This shift is toward manufacturing products that can deliver more than what the traditional treatments currently provide. Combination products are one type of treatment option that can meet the more rigorous demands of today’s patients.
Definition of a Combination Product
Combination products are usually therapeutic and diagnostic products that combine drugs, devices or biological products. They consist of two or more regulated components. Each drug, device, and biological product included in a combination product is referred to as a constituent part of the combination product.
They can include several combinations such as:
- Drug/device
- Biologic/device
- Drug/biologic
- Drug/device/biologic
Contact us by submitting a business inquiry online. We will get back to you very soon.
Examples of Combination Products are metered-dose inhalers co-packaged with a filled drug product cartridge, vaccines supplied as a pre-filled syringe and drug-eluting stents.
The Growing Demand for Combination Products
Patients are demanding more innovative drugs which can help cure their ailments. Modern day patients are insisting on speedy delivery of therapeutics, ease of use and affordability. These demands made the drug companies to focus on the invention and production of Combination Products.
Combination Products are now integral to the drug manufacturing industry. According to the Globe Newswire Global Forecast, the Drug Device Combination Product market is expected to reach 177.7 billion USD by 2024.
Given this forecast, the manufacturing companies have a responsibility to safely meet patients’ expectations, continuously improve and monitor the production and use of these products.
Safety Reporting for Combination Products
As the demand for combination products increases and more products are introduced into the market, it has become a growing concern for National Competent Authorities (NCAs) and Pharma companies to report and track device malfunction adverse events. There are several cases where the FDA has issued many warning letters to pharmaceutical and device manufacturing companies to maintain safety and quality in their systems. For instance: Recently, the cardiac implants of a Medical Device giant received alerts from FDA about two dangerous security flaws affecting a number of implantable devices.
Such instances are a Heads Up for Global Drug Safety Departments to:
- Take up extensive safety processing for combination products to ensure effective compliance.
- Create separate departments logging quality complaints and performing initial and final review
- Monitor recordkeeping requirements that enable safety data archiving
- Conduct continuous research and progressive development to fulfill patient needs
In today’s highly competitive and informative scenario, there is a strong focus on Postmarketing Safety Reporting (PMSR) requirements for Combination Products. Increased efforts have been made to understand the evolving global regulations and to determine the best compliance strategies.
Reporting and Tracking of Adverse Events
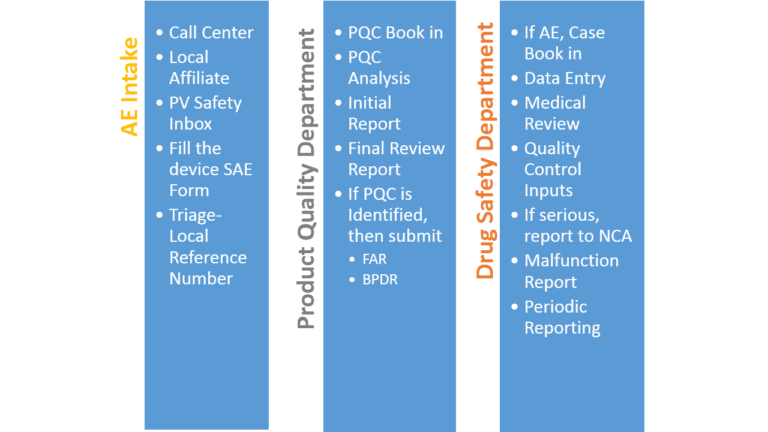
Marketing and Regulatory Requirements for Combination Products
Based on the type of scenario or event received, drug companies have to submit different reports to Notified bodies. Some of the prominent reports are 15 day reports, malfunction reports, 5 day reports, periodic reports, field alert reports and biological product deviation reports (BPDRs).
Companies also need to submit several applications to the relevant agencies before they can market their combination products.
The Marketing Applications required for Combination Products are:
- NDA – Report to CDER
- BLA – Report to CBER
- PMAs- To legally sell CLASS III
- PDPs
- HDEs
- De Novo Classification Requests (De Novos)
- 510(k)s – To legally sell CLASS II
Assigning Combination Products for Review
A Combination Product is assigned to a particular FDA lead center that has primary jurisdiction for its premarket review and regulation. The Primary Mode of Action (PMOA) of a combination product is determined before its assignment to a FDA center as required under section 503(g)(1) of the FD&C Act.
Section 503(g) defines the PMOA as ‘the single mode of action of a combination product that provides the most important therapeutic action of the combination product’.
Even after a combination product is assigned to a lead center, all applicable centers coordinate with each other and the Office of Combination Products for post market safety reporting if the need arises.
General Considerations for Combination Product PMSR Compliance
FDA issued the final rule on postmarketing safety reporting (PMSR) requirements for combination products on December 20, 2016 (81 FR 92603). The rule, as codified in 21 CFR 4, Subpart B, describes the method of compliance with PMSR requirements for combination products that have received FDA marketing authorization.
The final rule addresses combination products that are subject to premarket review by FDA. The entities subject to the final rule are ‘Combination Product Applicants’ and ‘Constituent Part Applicants’
The most important aspects of the final rule are:
- Application Type-Based Reporting Requirements: These requirements apply to both Combination Product Applicants and Constituent Part Applicants, based on the application type under which the combination product or constituent part has received marketing authorization.
- Constituent Part-Based Reporting Requirements: These requirements apply only to Combination Product Applicants and are based on the types of constituent parts included in the combination product.
- Information Sharing. Constituent Part Applicants have to share certain postmarketing safety information which they receive with each other.
- Submission Process: The final rule specifies the exact process of submitting Combination Product and Constituent Part Applicants PMSR information to the Agency.
- Streamlined reporting: The rule specifies certain ways to meet specific reporting requirements together in the same report.
- Records Retention: The rule specifies the exact records that should be maintained by Combination Product and Constituent Part Applicants and for how long.
Mandatory Information to be included in Combination Product PMSR Reports
The Combination Product PMSR Reports should include the following information:
- Combination Product Identifier: Clearly indicate that the report is for a combination product.
- Report Type: Mention the type of report for all the reports that are submitted. If one submission is made to cover multiple reporting requirements, identify each report type.
- Patient Identifier: Provide a patient identifier. If there is no patient involved in the event, enter ‘None.’
- Reporter Identifier: Provide the identifier for the individual who provided the report to the Combination Product Applicant.
- Suspect Medical Device: Include the procode that matches with the constituent parts of the device along with the device common name and/or brand name as applicable.
- Suspect Drug or Biological Product: Specify the known product attributes for the drug or biological product constituent parts. Include these drug or biological product attributes even if the drug or biological product constituent part was implicated in the event or not. For NDA/ANDA/BLA approved combination products, include the combination product’s application number in this field.
- Adverse Event Coding:
- For Device Application combination products, enter at least one Patient Problem Code, or a descriptive term if there is no code.
- For NDA/ANDA/BLA combination products, enter Reaction/Event Coding. Always use MedDRA (Medical Dictionary for Regulatory Activities) terms.
- Device Problem Code. Identify at least one device problem code.
- Cross-Reference to Other Reports: Mention the details of similar reports of international standards.
Future of Combination Products
More and more companies are collaborating to produce Combination Products. These partnerships provide patients with more innovative and affordable options.
According to informed surveys, approximately one-third of all medical products in development today are combination products. The field is an exciting one for device makers, not only because of the business opportunities, but because combination products have the ability to transform medicine as they have the potential to reverse diseases and not merely slow or stop the progression of diseases.
The growth factors for combination products come from many different sources. The most important among them is the fact that combination products can extend the patent life of a drug. When a drug’s patent is about to expire, it can be combined with a device or biologic to create a new product, with a new patent protection timeframe. Another strong factor is that there are less formal regulations governing combination products because the field is relatively new.
The availability of numerous products and technologies that can be combined to form combination products is tremendous. Exponential growth in this area is definitely on the cards as more products and technologies are developed and approved.
References
https://www.ivtnetwork.com/article/combination-products%E2%80%94engineering-future-healthcare
Postmarketing Safety Reporting for Combination Products Final Guidance –
https://www.fda.gov/media/111788/download
https://www.fda.gov/combination-products/about-combination-products



