- September 20, 2021
- Posted by: Techsol Life Sciences
- Category: Clinical Trials
Introduction to clinical trials site selection
Site selection is a comprehensive process that involves analysing sites, patient community, investigator profiles and site infrastructure for conducting a successful clinical trial. Pharma companies often look for alternative site discovery strategies to deal with the constant demand in trial sites globally. A data-driven approach across the clinical trial process offers an opportunity to harness the information for an enhanced clinical trial. Applying analytics with the right criteria and volume of data received from electronic health records (EHRs), drug and medical devices, sales/distribution channels will enable a patient-centric approach for an effective clinical trial.
When choosing a suitable trial site, there are a number of data driven factors that require careful consideration which includes the following:
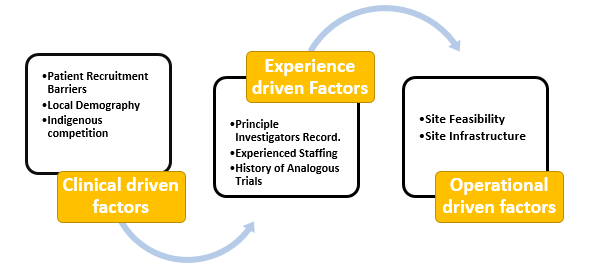
a. Clinical driven factors
b. Experience driven factors
c. Operational driven factors
Contact us by submitting a business inquiry online. We will get back to you very soon.
Clinical driven factors
Patient Recruitment Barriers: Sites that specialise in a specific target disease are less likely to experience difficulties recruiting patients or implementing the trial protocol. It is essential to understand the patient’s status in the current disease investigation. It thus becomes imperative to know whether
- Does the site currently have patients for the disease under investigation on their lists? If so, how many?
- And how many of them do they plan to enrol in your trial?
- Does the site have any competing trials?
- When will the on-going study finish and will those patients be eligible for inclusion in your study?
The above information is critical to decide whether the site’s involvement in any on-going studies should exclude involvement in your study.
- Local demography: Time, cost and inconvenience of travel will impact on subject recruitment and retention. Finding sites close to your target population is key when estimating how quickly you will realise enrolment targets. It is generally viewed that the more visits a study needs, the closer their target population should be to the site. The importance of travel burden on recruitment and retention increases the impact of a disease or condition has on the patient.
- Indigenous competition: The presence of only three sites in a particular city will most likely see sites competing for the same patient population. However, In some countries, several centres of excellence are located close together because they are the only centre in that country that treats the condition.
Experience-driven factors
- Principal Investigator’s record: Investigators actively involved in research in the proposed field are more likely to early study completion, and may be able to identify other great sites.
- Experienced staffing: It is essential to identify experienced staff at the site to monitor the trial and ensure protocol compliance. Their level of experience both with the protocol-defined procedures and study conduct (e.g., maintaining investigator files, completing eCRFs etc.) should be assessed.
- History of analogous trials (size and complexity): Review site’s history in conducting trials to ensure it has previously been involved in a study similar to yours. Analyse the previous patient enrolment data to gain insights and estimate the site’s recruitment capacity. Also inquire whether there have been any major changes at site that may affect the proposed study (e.g., changes in key personnel).
Operational driven factors
- Site feasibility: The CROs perform the site feasibility assessment as a precursor step to identify and close the decision of study placement. A robust and objective assessment is generally accepted as the best way to identify the feasible sites. There are commercial databases that provide data on patient population densities for key diseases. Some also provide more specific data such as past performance of key sites and country selection (e.g., IMS Health’s Study Optimizer and Pharma intelligence’s Site Trove and Trial. Once the CRO has been selected it is usually followed by a more rigorous feasibility assessment – using lists of possible sites/investigators who are invited to provide information about their capabilities, contractual processes, capacity and budget requirements. This process is often carried out in the form of questionnaires which is sent to sites to identify interest and collect relevant information including an estimate of the number of subjects they might expect to recruit.
- Site infrastructure: It is essential to know if the site has appropriate facilities and specialized equipment in order to perform clinical studies effectively. Thoroughly check whether the site has the required infrastructure to fulfil all the activities specified in the protocol. In case of the absence of such equipment, it helps in identifying the costs or logistics of providing that equipment. It in turn helps in analysing the timescales in study initiation or trial conduct if it relies on other departments in the hospital (e.g., pharmacy, imaging etc.)
Digitising the clinical trial site selection process leads to better outcomes
Site selection is a tedious manual process, digitising it will have a profound influence on the study outcomes by evading sub-optimal site choices that result in significant costs and delays.
The volume of data received is humongous, and these data assets are not centralized and are distributed across channels in multiple formats. Big data technologies enable organizations to amalgamate and standardize these assets into a single unified platform to allow uniform processing of data and provide valuable insights from the collected data. By Compiling data from globally acclaimed clinical registries, we have sourced and developed a customizable site selection criteria that enables appropriate site selection according to your preferences/ specific therapeutic areas / geographic location, PI profile / accreditations / certifications etc. Our global investigator database comprises of information about more than 2500 sites using which we can assist you to identify and select the most relevant site for your clinical trial.
Here’s how adopting a data-centric approach shall add value to the industry:
- Effective subject enrolment: Through data analytics on patient EHRs, pre-screening is performed for a given set of population. This drastically reduces the overall time for a clinical trial.
- Better decision making: Pharmaceutical companies prefer accurate data. Analysing that accurate data can help companies drive better data-driven decisions and clinical trial outcomes
- Constant Data monitoring: By constantly monitoring the required trial data, make faster data-driven decisions in site selection.
Conclusion
The paradigm shift in the way clinical research is evolving is anticipated to evolve further. Analytics along with artificial intelligence (AI), NLP and machine learning (ML) will drive the future of clinical research. However, what has been commissioned so far is just the tip of the iceberg seeing the volume of data available. Clinical analytics and emerging technologies will play a key role in driving efficiency and accelerating clinical trial process. There are clinical trial feasibility tools that evolve with ever-growing business needs. Companies need to make sure they find the one that works best for them.



