- September 2, 2021
- Posted by: Techsol Life Sciences
- Category: Pharmacovigilance

Aggregate reports are the cumulative reports in the database that emphasise on evaluation of safety profile and risk-benefit analysis of a drug. Aggregate reporting is the process of compilation and submission of aggregate reports to the regulatory agencies over the period of the product’s life cycle (during pre-marketing and post-marketing phase) to provide a comprehensive view of the drug’s safety profile.
Why Aggregate Reporting?
Aggregate reports are intended to provide an evaluation of the benefit-risk analysis balance for submission by pharmaceutical companies to the regulatory authorities at a defined point of time during the post-authorization phase. But why is aggregate reporting essential?
As greater number of individuals are exposed to the drugs compared to clinical trials, it is essential that real-world drug safety information is gathered in the post-marketing phase. During this stage, rare AE (Adverse Events) that haven’t been identified earlier might come to light. Medicinal products administered in real-world patients with underlying diseases tend to have various reactions. When submitted through Aggregate reports, such information will prove crucial for further studies on deciding the limitations of the product.
Contact us by submitting a business inquiry online. We will get back to you very soon.
Moreover, the post-marketing studies conducted to demonstrate drug effectiveness and risk stratification can lead to discovery of deviations in the Benefit-Risk balance of medicinal products. But it isn’t logical to conclude it and continue/ discontinue the medicine without further study. Therefore, uninterrupted surveillance of the benefit-risk profile of the product is necessary. Comprehensive and critical analysis of novel and evolving information on the risks and evidence of benefits must be identified and reported all of which is clearly reflected in aggregate reports.
Classification of Aggregate Reports
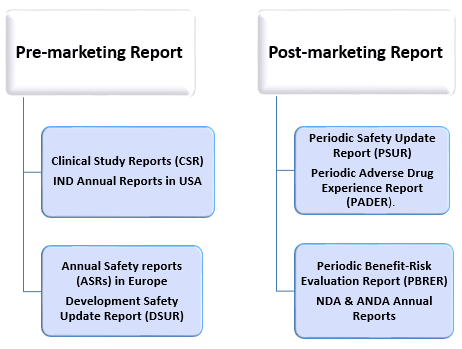
Aggregate reports are required during both pre-marketing and post-marketing stages of a product. These aggregate reports are required during both pre-marketing and post-marketing stages of a product. Each of these reports identifies and highlights a specific aspect of risk or benefit. The aggregate reports for respective stages are listed below.
Pre-marketing Aggregate Reports:
- Clinical Study Reports (CSRs)
- IND annual reports in the USA
- Development Safety Update Report (DSUR)
- Annual Safety Reports (ASRs) in Europe
Post-marketing Aggregate Reports:
- Periodic Safety Update Report (PSUR)
- Periodic Benefit-Risk Evaluation Report (PBRER)
- Periodic Adverse Drug Experience Report (PADER)
- NDA and ANDA Annual Reports
Challenges in Aggregate Reporting
Owing to the nature of the process, companies often face multiple challenges while compiling the aggregate reports and submitting them compliantly. Most commonly noticed, the documentation process is often quite daunting considering the extensive range of reports that must be included in the submission. While moving to electronic platforms has created some ease in sorting the reports, it still remains complex.
Similarly, scheduling and assigning the tasks among the workforce by identifying the right resource for individual process remains a long-standing challenge that must be addressed. Even after having allocated the right resources, aggregate reporting continues to remain labour-intensive manual process, and tracking it via spreadsheets might make it more disarrayed.
Any updates to the reports must be uniform. Therefore timely updates of information from multiple stakeholders such as the regulatory team, safety and clinical team, and marketing team are required. Pharma companies are required to track the status of each report from submission to approval. Considering the gamut of data involved, they must also identify and review the line-listings for accuracy.
The high volumes of data involved in the reporting process continue to increase with each passing day. And with it, the risks for errors leading to non-compliance findings is a major reason for concern for the pharma companies.
Amidst all of these challenges, another common concern is that of regional regulatory requirements. From time to time, the regulatory guidelines are revised in phases and they are not uniform across the globe pushing the need for multiple trackers for multiple products and countries.
Companies continue to realize that some of these challenges can be addressed by harmonization of guidelines which the global regulatory bodies continue to work on. Meanwhile, adapting to intelligent solutions that are cloud-enabled and can be sourced instantly irrespective of geographic limitations and varying timelines could alleviate a major set of issues. They allow for better coordination and instant data access. Improve search criteria and sorting ability cuts down on the time required for filing the data for submission.
Compier Aggregate Reports
Amidst the growing complexity involved in aggregate reporting, pharma companies will find that automating the regulatory reporting lifecycle management could offer improved quality in authoring and will help to complete the review in time-bound scenarios providing respite to the stakeholders. To help companies achieve the same, Techsol is offering Compier Aggregate Reports, a key module of our Compier platform focused on pharma operations oversight and compliance governance.
Key Product Features
- Report Schedule Management
- Predefined Templates for PADER, PSUR,
- PBRER, DSUR, CTPR & others as per business requirement
- Digital Authoring
- Collaborative Review
- Workflow driven activities
- Regulatory tracking
- Access Control
- Version Control
- Auto Update Rules
- Sharing Aggregate Reports
- Alerts & Notifications
- Insights & Analytics
If you want to understand how integrating Compier Aggregate Reports module can help you achieve digitalization and automation in associated processes or have queries with any other aspects of PV, please feel free to contact us at info@techsollifesciences.com.



